The market was hit by the shockwave of President Donald Trump’s long-telegraphed protectionist and drug price control policies finally entering the execution phase.
The primary drivers shaking the market were a "triple whammy" △the official launch of TrumpRx △the threat of a 25% tariff on Korean made pharmaceuticals, and △the U.S. FDA’s crackdown on unapproved generic weight-loss drugs.
Analysts suggest that the ‘Trump Risk,’ which had previously been confined to declarative threats on social media, has now maximized fears over rising operating costs for export-oriented firms as it translated into actual website operations and regulatory enforcement.
Amidst this external turmoil, Samil Pharmaceutical saw its stock price crash due to the effective suspension of its flagship product. In contrast Oscotec which saw the passing of its founder and major shareholder met a surprising reversal with a stock price increase driven by the market’s cold yet calculated anticipation of "governance changes."
|
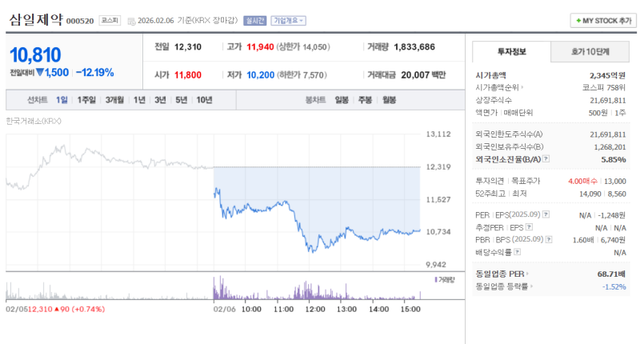
◇Samil Pharmaceutical 12.19% Plunge as 20-Year Staple ‘Glptide’ Faces Withdrawal
Samil Pharmaceutical closed at 10,810 won, down 12.19% from the previous trading day, fueled by heavy selling from foreign investors on the 6th. Volatility peaked as the price dipped as low as 10,200 won during intraday trading.
The collapse was triggered by a recommendation from the Ministry of Food and Drug Safety (MFDS) on the previous afternoon to suspend the use and prescription of ‘Glptide (Sulglicotide),’ a traditional "cash cow" for Samil used to treat gastric and duodenal ulcers/gastritis, citing its failure in an efficacy re-evaluation.
After reviewing clinical data under the latest scientific standards, the MFDS concluded that the drug’s therapeutic effects could not be statistically proven. While there were no safety issues, the failure to prove efficacy via domestic clinical trials led to the distribution of a safety letter recommending alternative prescriptions.
This is being analyzed as a de facto “death sentence” for the drug, which had been a steady seller in the gastroenterology market for over 20 years since its launch in 2005.
Glptide is a pillar of Samil’s gastroenterology division, and investors reacted sensitively because the product accounts for a significant portion of the company’s revenue structure approximately 5% of its 2025 revenue (estimated at 219.3 billion won).
As a self manufactured product, it boasts higher margins than licensed-in drugs thus, the loss of over 10 billion won in sales is expected to directly hit the 2026 operating profit margin.
An industry insider noted “The fact that a main product from a traditional mid-sized pharma failed a clinical re-evaluation raises doubts about the company’s overall R&D validation capabilities.”
◇Genome & Company: A Painful Fall where Policy Risk Meets ‘Underlying Weakness’
Genome & Company also fell 10.12% to close at 7,550 won, recording the second-largest drop in the sector after Samil. Experts analyzed this as the result of a regulatory storm directly hitting a company whose "foundational strength" had already been weakened by the loss of its main pipeline.
The launch of TrumpRx sent shockwaves through research-oriented bio-ventures. The U.S. government's declaration to intervene in distribution and cut manufacturer margins created uncertainty for ventures with no current revenue, suggesting a future where even successful development might not lead to profitability.
This, combined with the 25% tariff threat and the FDA’s crackdown on generics, dragged down the expected value of the company’s microbiome and ADC (Antibody-Drug Conjugate) technologies.
The reason Genome & Company responded more sensitively than others lies in its "underlying weakness" the announcement on the 4th of last month that it would abandon the Phase 3 clinical trial of its main pipeline, ‘GEN-001.’ Having already surrendered its core asset, the company lacked a "safe haven" to defend its valuation against external shocks.
Furthermore the pivot to the ADC business is viewed with skepticism as it is already a "red ocean" dominated by global big pharma and high interest rates (Warsh Shock) continue to magnify financial risks for bio-ventures in urgent need of capital.
◇Oscotec 3.77% Rise Despite Founder’s Death… The ‘Leclaza’ Value Reversal
In a sharp contrast to the sector's overall slump, Oscotec closed at 52,300 won, up 3.77%. This was driven by a combination of expectations for governance restructuring/M&A potential following the founder's death and the substantial asset value of ‘Leclaza.’
Founder and major shareholder Kim Jung-keun passed away in the U.S. on the night of the 5th. While the death of a major shareholder is typically an uncertainty risk, the capital market coldly evaluated it as an opportunity for a "valuation re-rating."
The focal point is the massive inheritance tax, estimated at around 140 billion won. With the bereaved family unlikely to pay this in cash, the market is betting on a scenario where they sell their stakes or pursue a total M&A of the company. Oscotec is considered a "highly coveted target" for conglomerates that have designated bio as a new growth engine, as it offers immediate and substantial royalty income.
The fundamental strength comes from ‘Leclaza (Lazertinib),’ a globally proven anti-cancer drug. Already FDA-approved and sold in the U.S. through Johnson & Johnson (J&J), Leclaza is expected to bring in massive royalties starting in 2026.
This elevates Oscotec from a venture relying on "dreams" to a company generating "actual profit," acting as a shield against Trump’s drug price pressures. Even if overall prices drop, Leclaza’s superior clinical data and J&J’s marketing power allow it to expand market share and preserve revenue, setting it apart from the export risks faced by smaller bio-ventures.
Copyright ⓒ 이데일리 무단 전재 및 재배포 금지
본 콘텐츠는 뉴스픽 파트너스에서 공유된 콘텐츠입니다.