|
Curacle is raising expectations for a potential technology transfer following the success of its Phase 2b clinical trial of CU01, a treatment for kidney disease, and the filing of a new use patent.
Cellbion is drawing attention for both early commercialization and possible out licensing opportunities based on its conditional approval application and Phase 2 clinical results for its radiopharmaceutical therapy (RPT) for prostate cancer.
Meanwhile, NGeneBio has entered into a partnership with LG AI Research to commercialize a pathology AI-based “one-minute diagnosis” solution accelerating its transformation into an AI medical data company.
Although the three companies are pursuing different strategies, they share a common denominator each has secured a clear commercialization catalyst through clinical results, patents or technology alliances. Market observers note that these firms are now moving beyond the pure R&D stage and into a phase of tangible value creation.
◇Curacle’s CU01 Eyes Licensing Deal
Expectations surrounding Curacle’s kidney disease drug candidate CU01 are rapidly gaining momentum. Following the successful completion of its Phase 2b clinical trial demonstrating both efficacy and safety, and the filing of a new use patent CU01 is increasingly being viewed as having moved beyond a mere development-stage asset into a full-fledged commercialization phase.
According to KG Zeroin MP Doctor (formerly MarketPoint) on the day, Curacle’s shares closed at 9,720 won, up 9.34% (830 won) from the previous trading session.
On January 20, Curacle announced that CU01 had demonstrated both efficacy and safety in a Phase 2b clinical trial involving 240 patients with diabetic nephropathy across 24 hospitals in Korea, meeting its primary endpoint by improving uACR while showing a trend toward maintaining eGFR.
Based on these results the company completed the filing of a use patent on January 27, following an earlier provisional application. If the patent is granted CU01 is expected to secure global market exclusivity in the treatment of diabetic nephropathy for the next 20 years.
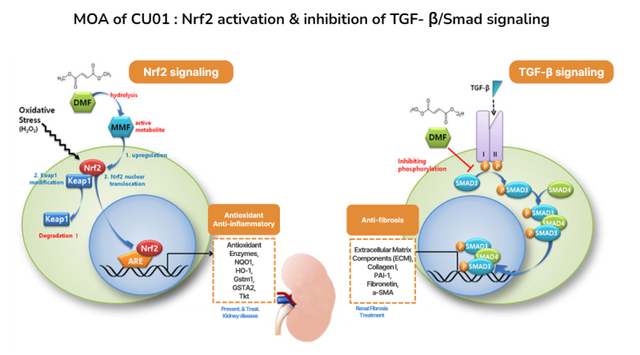
CU01 is an oral therapy based on dimethyl fumarate (DMF) and exerts anti-inflammatory, antioxidant, and anti-fibrotic effects through activation of Nrf2 and inhibition of TGF-β signaling.
DMF has already been used for many years in the treatment of psoriasis and multiple sclerosis with its safety and efficacy well established. Curacle is leveraging this proven compound to expand its application into the field of renal diseases.
A company official said “By demonstrating both efficacy and safety in the Phase 2b trial, we have significantly increased the possibility of commercialization as a new kidney disease therapy. Moreover, with the filing of this use patent CU01 has gained additional exclusive rights for this specific indication on top of its existing patents further enhancing its business value.”
The official added “This patent is expected to serve as an important catalyst not only in the domestic market but also in global licensing negotiations, and based on this, we will move forward in earnest with the commercialization of CU01.”
|
◇Cellbion Nears Conditional Approval, Challenges Pluvicto
Expectations surrounding Cellbion’s radiopharmaceutical therapy (RPT) for prostate cancer are rising rapidly as the company draws attention for both the possibility of early commercialization following its application for conditional approval and potential out licensing based on its Phase 2 results. On the day, Cellbion’s shares closed at 26,300 won up 7.35% (1,800 won) from the previous session.
Cellbion received the final clinical study report (CSR) for its Phase 2 trial in metastatic castration-resistant prostate cancer (mCRPC) last month and subsequently submitted an application for conditional approval to the Ministry of Food and Drug Safety.
The conditional approval pathway allows drugs for rare and intractable diseases to be marketed based on Phase 2 data alone.
In the Phase 2 trial, the therapy achieved an objective response rate (ORR) of approximately 35.9%, demonstrating superior efficacy compared with leading competing RPT therapies. The company aims to obtain conditional approval in the first half of the year and begin patient supply in the second half. Cellbion is also conducting a combination trial with MSD’s Keytruda.
A company official said it is pursuing indication expansion and out-licensing as core strategies and plans to advance licensing discussions based on the Phase 2 data, noting that the industry estimates the out-licensing value of 177Lu-Focuvotide at more than 1 trillion won.
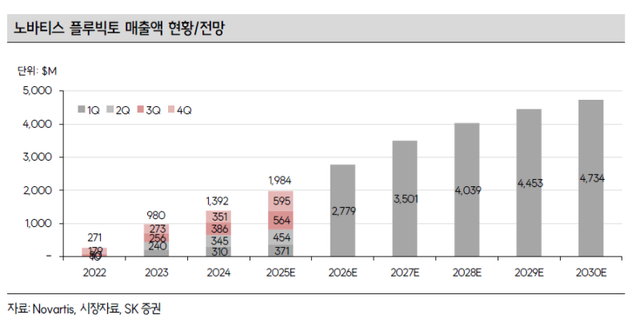
In clinical studies, Focuvotide has shown advantages over Novartis’ Pluvicto in both efficacy and safety, while also offering better price competitiveness, with the cost per treatment set at 27 million won compared with Pluvicto’s 35 million won in Korea.
|
◇NGeneBio, LG AI to Launch One-Minute Diagnosis
NGeneBio continued to gain momentum following news of its collaboration with LG AI Research with its shares closing at 2,245 won, up 11.41% from the previous session.
On January 20, the company announced that it had signed a licensing agreement for LG AI Research’s precision medicine AI model, EXAONE Path 2.0 describing the deal as a key step in its transformation into an “AI medical data company.”
Under the agreement NGeneBio will integrate an AI model that predicts EGFR mutations into its platform. Based on pathological tissue images, the technology can determine EGFR mutation status in non-small cell lung cancer patients in less than one minute, compared with about two weeks required by conventional genetic testing.
NGeneBio plans to begin clinical validation of the model with Asan Medical Center and, after obtaining approval from the Ministry of Food and Drug Safety (MFDS) as a Software as a Medical Device (SaMD) to deploy it across its platforms including NGLIS and NGAS which are already used by major hospitals in Korea.
CEO Min sik Kim said the partnership marks a turning point in the company’s data strategy and will accelerate its evolution beyond an NGS solutions provider into an AI medical data company.
Copyright ⓒ 이데일리 무단 전재 및 재배포 금지
본 콘텐츠는 뉴스픽 파트너스에서 공유된 콘텐츠입니다.