Alteogen the largest company by market capitalization on the KOSDAQ, led the downturn pulling the index down 2.57% to close at 951.29. Industry watchers say the episode is being viewed not as an isolated corporate issue, but as an event that has undermined market trust itself.
|
◇Royalty Gap Exposed at 2% Trust Damage Outweighs the Numbers
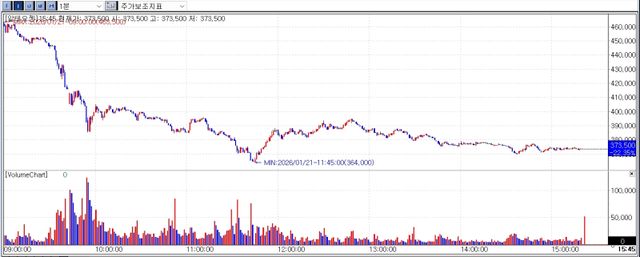
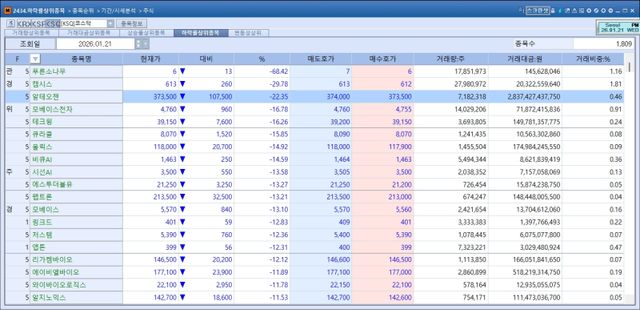
According to KG Zeroin’s MP Doctor, Alteogen shares fell 22.35% to won 373,500. The sell-off followed confirmation that the royalty rate was set at 2%, well below the 4~5% level the market had widely anticipated.
Selling pressure intensified after it became known that Merck’s (MSD) Form 10-Q for the third quarter of 2025 explicitly stated a 2% royalty on net sales for Keytruda Qlex, the subcutaneous (SC) formulation of Keytruda.
Keytruda Qlex incorporates Alteogen’s ALT-B4 technology. Keytruda remains the world’s top selling immuno oncology drug, generating USD 29.5 billion in 2024 sales. While the sales based milestone for the SC formulation up to USD 680 million was largely in line with market expectations the confirmed 2% royalty rate came as a shock.
Assuming a 50% SC conversion rate, Alteogen’s annual royalty income is estimated at around won 430 billion far below earlier expectations based on a 5% royalty that implied annual income exceeding won 1 trillion.
Following the plunge Alteogen issued a statement reiterating that royalty and milestone terms remain confidential under its contract with MSD. The company emphasized that it can receive up to USD 1 billion in milestones before transitioning to royalties and that royalties could be collected for up to 18 years until patent expiry in early 2043.
However uncertainty over when royalty payments would actually begin continued to weigh on investor sentiment.
|
◇Peptron Déjà Vu The Cost of Failed Expectation Management
The fallout quickly spread across the biotech sector, with many drawing parallels to the Peptron case. Peptron had surged on expectations of a full licensing deal with Eli Lilly following a platform technology evaluation agreement only to later revise the evaluation period from “about 14 months” to “up to 24 months” triggering confusion and disappointment.
On the same day Peptron shares dropped more than 13%. Industry sources noted that, in both cases, companies may have withheld material information they were already aware of, only disclosing it after market expectations had peaked. “When expectations are built up and timelines are later revised, investors inevitably feel betrayed.” one industry insider said.
Alteogen had long been regarded as one of the more trusted names in Korea’s biotech space, thanks to its platform based licensing model reduced clinical risk exposure, and multiple partnerships with global pharmaceutical companies tied to blockbuster drugs such as Keytruda.
This perception made the current episode particularly damaging. The broader loss of confidence was reflected in the day’s top KOSDAQ decliners which included not only Alteogen and Peptron but also other biotech names such as Olix, LigaChem Bio, ABL Bio, Y-Biologics, Alginomics and G2GBio.
|
◇SAMIK PHARM, Slides on Encapsulation Illusion Criticism
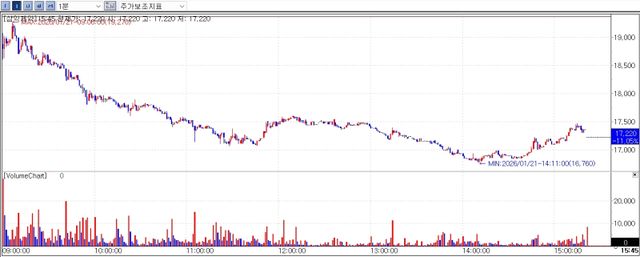
Meanwhile SAMIK PHARM closed at won 17,220 on the day down 11.05% from the previous session amid the impact of PharmEdaily’s premium content. The company had previously seen its shares surge by nearly 50% after announcing the registration of a patent for its long acting injectable platform technology.
PharmEdaily pre-released a premium article titled “SAMIK PHARM The Long Acting Platform That Lifted Shares 50% A Closer Look Reveals Weak Technical Competitiveness” at 8:10 a.m. on January 21, before making it freely available at 11:00 a.m. the same day.
According to the report SAMIK PHARM. disclosed a maximum encapsulation efficiency of 95.4% in its patent filings but the figure showed wide variability depending on drug loading levels.
As drug loading increased encapsulation efficiency fell sharply to 73.3%, a drop of more than 20 percentage points. Industry experts noted that such large fluctuations in encapsulation efficiency suggest that sufficient technical stability has yet to be secured.
By contrast, competing companies such as Peptron, Inc., Inventage Lab, and G2GBio were cited as maintaining encapsulation efficiencies of 80~90% across drug loading levels ranging from 10% to 60% indicating more stable performance even at significantly higher drug loads.
In SAMIK PHARM’s case analysts warned that relatively low drug loading may have created an optical illusion that made encapsulation efficiency appear higher than it would be under commercially relevant conditions.
An industry source commented “There appears to have been confusion between the concepts of encapsulation efficiency and drug loading, which may have misled investors.”
adding “Given that the technology is only at the patent registration stage, it should also be taken into account that substantial verification will be required before commercialization.”
Copyright ⓒ 이데일리 무단 전재 및 재배포 금지
본 콘텐츠는 뉴스픽 파트너스에서 공유된 콘텐츠입니다.