Shares of Samsung Biologics and ST Pharm climbed on expectations they could benefit if U.S. customers shift business away from Chinese competitors.
Meanwhile antibody drug conjugate or ADC names continued to surge led by AimedBio. Kyongbo Pharmaceutical jumped about 10% after announcing its entry into the ADC business.
|
◇Biosecure Act tailwinds
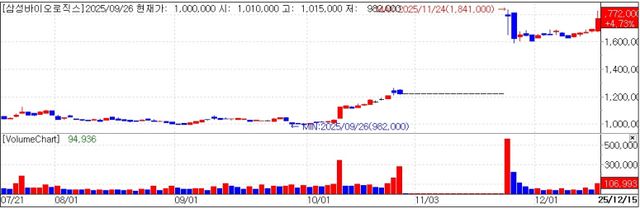
Samsung Biologics ended Monday up 4.73% at 1.772 million won, according to KG Zeroin’s MP Doctor data service. The stock touched 1.83 million won intraday before easing late in the session.
The rally was attributed to growing expectations that the Biosecure Act could pass and create “spillover” benefits for non Chinese CDMOs.
Industry sources said the bill was included in a compromise version of the final U.S. National Defense Authorization Act, or NDAA. The package passed the House with 312 votes in favor and 112 against. It still requires a Senate vote, and final approval would depend on a presidential signature. Market participants widely expect passage by year’s end.
The Biosecure Act would prohibit U.S. government agencies and companies receiving U.S. government funding from contracting with biotechnology firms the U.S. government designates as “of concern.”
The U.S. has previously named several China-based companies as firms of concern including WuXi Biologics, WuXi AppTec, BGI, MGI and Complete Genomics.
If the bill becomes law this year Chinese CDMOs would face major restrictions in the global market analysts said while South Korea and other countries could gain business diverted from China.
Samsung Biologics currently has combined production capacity of 784,000 liters across five plants, outpacing competitors such as Lonza, with 460,000 liters, and WuXi Biologics, with 456,000 liters, the report said.
The global antibody CDMO market has been shared by Samsung Biologics, Lonza, WuXi Biologics and Catalent but the report said implementation of the Biosecure Act could leave Samsung Biologics, Lonza and Catalent to capture much of the market.
The Bioeconomy Research Center at the Korea Bio Association said the NDAA compromise cannot be amended during the voting process, and must be passed within the year due to timing and procedural requirements making approval “highly likely.” The group added that China’s position in U.S. pharmaceutical CDMO services could be significantly weakened.
A Samsung Biologics official said interest has grown as several brokerages have released upbeat reports tied to the Biosecure Act.
Shares of Samsung Epis Holdings also rose sharply climbing 7.26% to 709,000 won the report said with the move attributed to expectations that Samsung Biologics could benefit from the legislation. ST Pharm gained 3.77% to close at 132,100 won.
◇ADC names remain in focus
AimedBio which listed on Dec. 4, surged 26.12% to close at 75,000 won. The stock is up about 540% from its 11,000 won IPO price, the report said.
The continued rally was attributed to rising investor interest in ADCs as a next generation therapeutic modality.
Genome & Company also has climbed on a shift toward ADCs with its shares up 52.87% so far this month according to the report.
Kyongbo Pharmaceutical, a subsidiary of Chong Kun Dang, rose 10.02% to 6,480 won after announcing it opened an “ADC Research Center” to produce preclinical research samples.
Kyongbo said the center is designed as an end-to-end ADC CDMO platform from drug substance, or DS, used for preclinical testing to what it described as the country’s first finished-drug, or DP, production line.
Full scale manufacturing is slated to begin in 2026. The company said a new ADC production plant under construction would enable one-stop supply including finished products for clinical use.
Kyongbo CEO Kim Tae young said the research center and production plant will form a one stop supply chain helping domestic ADC developers and biotech startups reduce the time and cost burden of producing clinical samples and contributing to South Korea’s pharmaceutical and biotech industry.
Copyright ⓒ 이데일리 무단 전재 및 재배포 금지
본 콘텐츠는 뉴스픽 파트너스에서 공유된 콘텐츠입니다.