NanoEntek also soared to the upper price limit after announcing plans to showcase a prototype of its automated high throughput cell-analysis robot at the world’s largest cell biology conference. Aptabio Inc. climbed sharply as well following updates that its Phase 2b trial for a diabetic kidney-disease treatment is progressing smoothly.
According to MP Doctor, formerly Market Point, Hyundai Pharmaceutical closed at 6960 won on Dec. 9, up 5.94 percent(390 won) from the previous session. The company posted limit-up gains for two consecutive days and touched 8290 won intraday marking another 52-week high.
Hyundai’s rally was driven by news that Italy-based Cosmo Pharmaceuticals had achieved successful Phase 3 results for clascoterone 5% solution a topical treatment for male pattern hair loss. Hyundai signed an exclusive license in agreement in 2023 with Cosmo subsidiary Cassiopea for the acne medication Winlevi(clascoterone) for the Korean market.
Cosmo announced on Dec. 5 that two Phase 3 studies conducted across 50 sites in the United States and Europe enrolled a total of 1,465 patients. The clascoterone group showed a 539 percent increase in hair count at the application area versus placebo. The results support the drug’s commercial potential analysts said.
Clascoterone, at lower doses, was approved as an acne medication and launched in the United States in 2020. It received Korean regulatory approval in September and is preparing for domestic launch. Hyundai Pharmaceutical secured the rights to the acne drug and the Phase 3 success for the hair-loss formulation appears to have influenced investor sentiment. However, Hyundai confirmed it has no licensing rights to the hair loss product.
“There is investor interest related to Cosmo Pharmaceuticals’ Phase 3 success in hair loss treatment.” a Hyundai Pharmaceutical official said. “While we hold the license for the acne indication we have no agreement related to the hair loss drug. The issue is unrelated to Hyundai.”
|
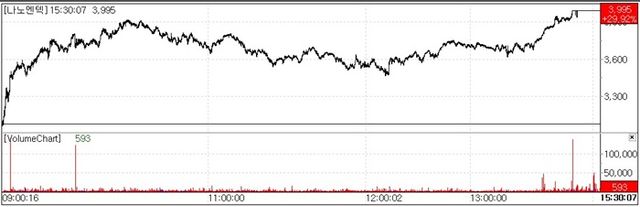
◇NanoEntek Surges on Debut of High Throughput Cell Analysis Robot
NanoEntek shares surged to the daily upper limit, closing at 3995 won, up 29.92 percent(920 won). It marks the stock’s first approach to the 4000won range in about 11 months following a high of 4140 won on Jan. 9.
The rally followed news that the company will introduce its next-generation bio robot, the EVE-HT A26, at Cell Bio, the world’s largest international cell-biology conference held Dec. 6–10 in Philadelphia.
The EVE-HT A26 is an all-in-one, fully automated platform that streamlines cell preprocessing, counting and analysis. Built with Bio-MEMS technology combined with linear-motor and precision robotic-pipetting systems, the seven-axis robot enables high speed and high accuracy operations. It can process 96 samples in 15 minutes without researcher intervention, allowing applications across cell biology, antibody therapeutics, cell therapies, infectious-disease diagnostics and clinical research.
The platform was reportedly developed at the request of major global pharmaceutical companies and is expected to draw strong demand in CDMO production workflows. NanoEntek plans to officially launch the product in the first quarter of next year.
“This is the world’s first automated platform specialized for cell counting” a company official said. “We expect it to become a game-changer in large scale production settings including CDMOs. Demonstrating the prototype at Cell Bio will help us engage global researchers and secure early references.”
◇Aptabio Gains on Smooth Progress of Phase 2b Diabetic Kidney-Disease Trial
Aptabio Inc. shares jumped 14.39 percent(1210 won) to 9620 won after the company reported that its Phase 2b trial of APX-115(isuzinaxib) a treatment for diabetic kidney disease, is proceeding as planned. The stock returned to the 9000won level for the first time in about four months.
The ongoing Phase 2b trial has completed dosing in about 30 percent of the 186 enrolled patients. Aptabio expects to finish dosing by year end and release interim results in the second half of next year. The steady trial progress has heightened expectations for clinical success and a potential license out deal.
APX-115 previously demonstrated efficacy in a European Phase 2a trial of 140 patients. Notably, in patients with moderate to severe disease(eGFR < 45), the urine albumin-creatinine ratio(UACR) decreased by about 50 percent versus placebo(P < 0.05), securing human proof-of-concept data.
The findings were selected for an oral presentation during the High-Impact Clinical Trials session at Kidney Week 2022. APX-115 was also chosen as a national drug-development project by Korea’s KDDF in June.
“With the cooperation of participating institutions, patient enrollment and dosing are progressing smoothly,” CEO Soo Jin Lee said. “We aim to clearly demonstrate treatment efficacy in the Phase 2b trial and advance global licensing and commercialization.”
Copyright ⓒ 이데일리 무단 전재 및 재배포 금지
본 콘텐츠는 뉴스픽 파트너스에서 공유된 콘텐츠입니다.